Merck the developer of pembrolizumab announced the approval. CPS 1 KEYTRUDA in combination with platinum and fluorouracil.
 Head And Neck Cancer Treatment Update Pembrolizumab Nivolumab And Pd L1 Markers Youtube
Head And Neck Cancer Treatment Update Pembrolizumab Nivolumab And Pd L1 Markers Youtube
So that is another possible option for you if the Keytruda doesnt work.
Keytruda head and neck cancer. Evidence-based recommendations on pembrolizumab Keytruda for untreated metastatic or unresectable recurrent head and neck squamous cell carcinoma HNSCC in adults whose tumours express PD L1 with a combined positive score CPS of 1 or. We would like to know if anyone has had this treatment what side effects you experienced and how effective it was. KEYTRUDA is a prescription medicine used to treat a kind of cancer called head and neck squamous cell cancer HNSCC.
Median progression-free survival PFS was 98 months. KEYNOTE-012 was an expansion of a phase 1b trial of Keytruda in squamous cell carcinoma of the head and neck. KEYTRUDA may be used with the chemotherapy medicines fluorouracil and a platinum as your first treatment when your head and neck cancer has spread or.
FDA approves pembrolizumab for first-line treatment of head and neck squamous cell carcinoma On June 10 2019 the Food and Drug Administration approved pembrolizumab KEYTRUDA Merck for the. Its the first immunotherapy drug approved for head and neck cancer. My husbands head and neck cancer has spread to his lungs and left adrenal gland.
KEYTRUDA may be used with the chemotherapy medicines fluorouracil and a platinum as your first treatment when your head and neck cancer has spread or. The approval of Keytruda gives. It sounds as if you are having a good result--no tumor symptoms and manageable Keytruda side effects.
The drug was initially rejected in January due to uncertainties in the clinical trial data but has now been approved by the National Institute of Health and Care Excellence NICE based on additional data. 67 of those users who reviewed Keytruda reported a positive effect while 33 reported a negative effect. Melanoma a skin cancer non-small cell lung cancer NSCLC a type of lung cancer classical Hodgkin lymphoma a cancer of the white blood cells urothelial cancer a cancer of the bladder and urinary tract a cancer affecting the head and neck.
Keytruda pembrolizumab may be more beneficial than standard therapy for patients whose head and neck cancer has spread or returned after a first round of chemotherapy. Keytruda is already approved for melanoma and non-small cell lung cancer. The US Food and Drug Administration FDA has approved Keytruda pembrolizumab to treat people with head and neck squamous cell carcinoma that has spread or come back after previous chemotherapy treatment.
The findings Pembrolizumab versus methotrexate docetaxel or cetuximab for recurrent or metastatic head. There is another immunotherapy drug that has been approved for HN SCC Opdivo. Treatment with Keytruda pembrolizumab extends in clinically meaningful ways the lives of people with recurrent or metastatic head and neck squamous cell carcinoma SCC compared to standard treatment especially those whose tumors express PD-L1 data from a Phase 3 trial show.
Squamous cell carcinoma that recurs or metastasizes is even more difficult and has become an area of substantial unmet medical need. An accelerated FDA approval has been granted to the immunotherapy Keytruda pembrolizumab for patients with recurrent or metastatic head and neck squamous cell carcinoma HNSCC following progression on a platinum-containing chemotherapy. Keytruda has an average rating of 68 out of 10 from a total of 9 ratings for the treatment of Head and Neck Cancer.
Cancer Indication PD-L1 Expression KEYTRUDA as a single agent is indicated for the first-line treatment of patients with metastatic or with unresectable recurrent head and neck squamous cell carcinoma HNSCC whose tumors express PD-L1 CPS 1 as determined by an FDA-approved test. And there are trials out there for other new drugs and combinations. Some people with head and neck cancer will now have access to the immunotherapy drug pembrolizumab Keytruda on the NHS in England.
Best of luck to you. KEYTRUDA is a prescription medicine used to treat a kind of cancer called head and neck squamous cell cancer HNSCC. Keytruda is a cancer medicine used to treat.
Head and neck cancer has long been a particular challenge to treat as it is critical that the functions of the head and neck be preserved while also treating the disease effectively. Our oncologist has offered Keytruda as a treatment option. User Reviews for Keytruda to treat Head and Neck Cancer.
In an interview with CURE Mehra discusses what the KEYNOTE-012 findings could mean for patients the growing role for the immunotherapy agent in head and neck cancer and what else is on the horizon for the treatment landscape. ORR and PFS were both above published data for Keytruda alone 146 and 20 months respectively. Thats the conclusion of a Phase 3 study Pembrolizumab pembro vs standard of care SOC for recurrent or metastatic head.
 Keytruda Pembrolizumab For Head And Neck Cancer Cancer Therapy Advisor
Keytruda Pembrolizumab For Head And Neck Cancer Cancer Therapy Advisor
Regulator Oks Keytruda For Head And Neck Cancer Therapy Pharma 기사본문 Kbr
 Fda Greenlights Merck S Blockbuster Drug Keytruda For First Line Treatment Of Head Neck Cancer
Fda Greenlights Merck S Blockbuster Drug Keytruda For First Line Treatment Of Head Neck Cancer
 Keytruda Precision Cancer Immunotherapy Treatment For Head And Neck Cancer Cancerconnect
Keytruda Precision Cancer Immunotherapy Treatment For Head And Neck Cancer Cancerconnect
 Head And Neck Squamous Cell Cancer L Keytruda Pembrolizumab Patients
Head And Neck Squamous Cell Cancer L Keytruda Pembrolizumab Patients
 Pembrolizumab Alone Or With Chemotherapy Versus Cetuximab With Chemotherapy For Recurrent Or Metastatic Squamous Cell Carcinoma Of The Head And Neck Keynote 048 A Randomised Open Label Phase 3 Study The Lancet
Pembrolizumab Alone Or With Chemotherapy Versus Cetuximab With Chemotherapy For Recurrent Or Metastatic Squamous Cell Carcinoma Of The Head And Neck Keynote 048 A Randomised Open Label Phase 3 Study The Lancet
 Msd S Keytruda Scores Another Eu Okay In Head And Neck Cancer Pmlive
Msd S Keytruda Scores Another Eu Okay In Head And Neck Cancer Pmlive
 Merck Co S Keytruda Shows Potential In Head And Neck Cancer Use
Merck Co S Keytruda Shows Potential In Head And Neck Cancer Use
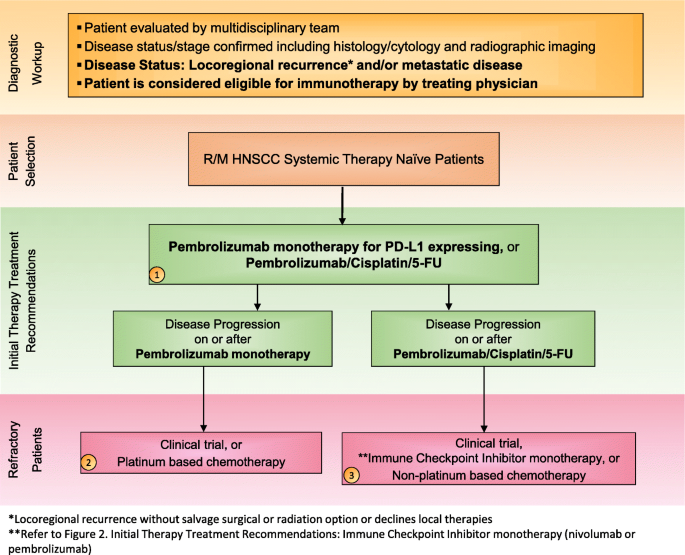 The Society For Immunotherapy Of Cancer Consensus Statement On Immunotherapy For The Treatment Of Squamous Cell Carcinoma Of The Head And Neck Hnscc Journal For Immunotherapy Of Cancer Full Text
The Society For Immunotherapy Of Cancer Consensus Statement On Immunotherapy For The Treatment Of Squamous Cell Carcinoma Of The Head And Neck Hnscc Journal For Immunotherapy Of Cancer Full Text

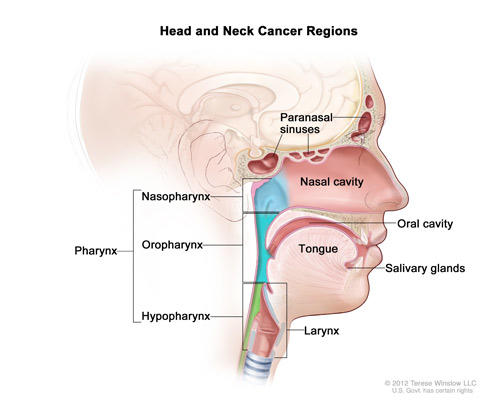 Fda Approves Pembrolizumab For Head And Neck Cancer National Cancer Institute
Fda Approves Pembrolizumab For Head And Neck Cancer National Cancer Institute
 Pembrolizumab Alone Or With Chemotherapy Versus Cetuximab With Chemotherapy For Recurrent Or Metastatic Squamous Cell Carcinoma Of The Head And Neck Keynote 048 A Randomised Open Label Phase 3 Study The Lancet
Pembrolizumab Alone Or With Chemotherapy Versus Cetuximab With Chemotherapy For Recurrent Or Metastatic Squamous Cell Carcinoma Of The Head And Neck Keynote 048 A Randomised Open Label Phase 3 Study The Lancet
 Clinical Trial Results In Head And Neck Squamous Cell Cancer Patients
Clinical Trial Results In Head And Neck Squamous Cell Cancer Patients
 Esmo18 Merck S Keytruda Alone And In Combo Makes Its Case For Earlier Use In Head And Neck Cancer Fiercepharma
Esmo18 Merck S Keytruda Alone And In Combo Makes Its Case For Earlier Use In Head And Neck Cancer Fiercepharma

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.