PADCEV may be used if you have. TOP - May 2021 Vol 14 No 3 - ASCO GU Cancer Highlights.
 Asco Gu 2021 Ev 201 Cohort 2 Enfortumab Vedotin In Cisplatin Ineligible Patients With Locally Advanced Or Metastatic Urothelial Cancer Who Received Prior Pd 1 Pd L1 Inhibitors
Asco Gu 2021 Ev 201 Cohort 2 Enfortumab Vedotin In Cisplatin Ineligible Patients With Locally Advanced Or Metastatic Urothelial Cancer Who Received Prior Pd 1 Pd L1 Inhibitors
The recommended enfortumab vedotin-ejfv dose is 125 mgkg up to a maximum dose of 125 mg administered as an intravenous infusion over 30 minutes on days 1 8 and 15 of a 28-day cycle until.

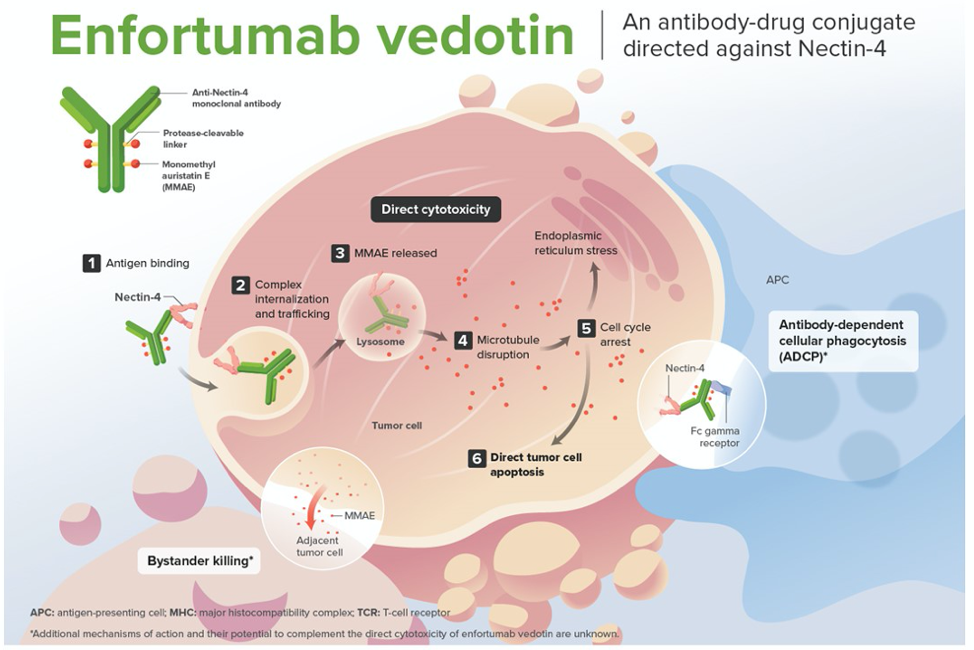
Enfortumab vedotin bladder cancer. Enfortumab vedotin is a first-in-class antibody-drug conjugate ADC that is directed against Nectin-4 a protein located on the surface of cells and highly expressed in bladder cancer4. Enfortumab vedotin-ejfv a first-in-class antibody-drug conjugate ADC that is directed against Nectin-4 a protein located on the surface of cells and highly expressed in bladder cancer was conditionally approved by the FDA in December 2019 based on the Accelerated Approval Program. However erdafitnib can only be used to treat cancers that have an alteration in one of several genes called FGFR.
If they improve they probably should not restart treatment with enfortumab vedotin Bladder cancer is an aggressive cancer stressed Ms. In patients with metastatic urothelial cancer who received previous therapy as well as in cisplatin-ineligible patients with this cancer enfortumab vedotin-ejfv Padcev improved overall survival OS according to new data presented at. Yu EY al.
Urothelial cancer a type of bladder cancer that is locally advanced or has metastasized. The antibody-drug conjugate ADC enfortumab vedotin-ejfv Padcev induced a response in over half of patients with advanced urothelial cancer who previously received a PD-1PD-L1 inhibitor but were ineligible for cisplatin according to findings from the pivotal phase 2 EV-201 trial. Enfortumab vedotin was the second targeted drug to be approved for the treatment of advanced bladder cancer in 2019.
Particularly the revealed information are for cohort 2 of the EV-201 examine by which the antibody-drug conjugate ADC enfortumab vedotin was administered to cisplatin-ineligible sufferers with. To briefly review enfortumab vedotin is an ADC specific for targeting Nectin-4 which is highly expressed in urothelial carcinoma. Enfortumab vedotin-ejfv is approved to treat.
Rosenberg noted some of those patients may need treatment in the ICU. New directions include erdafitinib a fibroblast growth factor receptor FGFR inhibitor in patients with corresponding mutations in FGFR23 receptor. Knowledge from the section 2 EV-201 trial supporting a possible expanded FDA approval of enfortumab vedotin Padcev in bladder most cancers have been revealed within the Lancet Oncology.
It is used in adults who have received platinum chemotherapy and either a PD-1 or PD-L1 inhibitor for locally advanced or metastatic disease. Lancet Oncol 11 May 2021. It has generally been treated aggressively with cytotoxic agents such as cisplatin which are challenging.
Although It is quite rare Dr. In this single-arm phase II study enfortumab vedotin shows promising anti-tumor activity in an unselected population of patients with mUC who have had either prior PD-1L1 or prior platinum therapy. It received FDA approval based on phase III data recently and thus represents an alternative to established third-line.
Based on the data from cohort 2 of EV-201 a supplemental Biologics License Application has been filed with the FDA to expand enfortumab vedotins approval to include patients with locally advanced or metastatic urothelial cancer who have been previously treated with a PD-1L1 inhibitor and are ineligible for cisplatin. Combining the antibody drug conjugate enfortumab vedotin Padcev with the immune checkpoint inhibitor pembrolizumab Keytruda showed encouraging results in patients with locally advanced or metastatic urothelial cancer who were unable to receive cisplatin-based chemotherapy in. These results for enfortumab vedotin indicate it may be able to help patients whose urothelial cancer progresses following treatment with standard chemotherapy and a PD-1 or PD-L1 inhibitor Common treatment-related adverse events with enfortumab vedotin included fatigue alopecia decreased appetite rash and peripheral neuropathy.
The cytotoxic agent in enfortumab vedotin. Enfortumab vedotin shows promise in cisplatin-ineligible patients. 1 The monoclonal antibody targeting Nectin-4 is connected by a linker molecule to a cytotoxic drug payload.
Enfortumab Vedotin Prolongs Survival in Patients with Metastatic Bladder Cancer. This was a high-risk population many with visceral metastases and despite this enfortumab showed great efficacy with a 12 CR rate. Enfortumab vedotin EV is an antibody-drug conjugate targeting nectin-4 and is conjugated with monomethyl auristatin E MMAE.
PADCEV enfortumab vedotin-ejfv is a prescription medicine used to treat adults with bladder cancer and cancers of the urinary tract renal pelvis ureter or urethra that has spread or cannot be removed by surgery. In the case of enfortumab vedotin the payload is monomethyl auristatin E MMAE a potent antimitotic agent that blocks the polymerization of tubulin. Earlier in the year FDA granted accelerated approval to erdafitinib Balversa.
