Food and Drug Administration FDA has approved FoundationOneCDx as the first companion diagnostic for larotrectinib Vitrakvi. Your therapeutic needs a companion.
 The Importance Of Companion Diagnostics
The Importance Of Companion Diagnostics
You need a resourceful partner.

Fda companion diagnostics. A companion diagnostic device can be in vitro diagnostic device or an imaging tool that provides information that is essential for the safe and effective use of a corresponding therapeutic product. Alpelisib Piqray in advanced or metastatic breast cancer rucaparib in advanced ovarian cancer and alectinib Alcensa in a specific type of NSCLC. Define in vitro companion diagnostic device hereafter referred to as an IVD companion diagnostic device Explain the need for FDA oversight of IVD companion diagnostic devices Clarify that in most circumstances an IVD companion.
A companion diagnostic CDx can transform the promise of personalized medicine into reality. FDA defined the term IVD companion diagnostic device and described certain regulatory requirements in the guidance entitled In Vitro Companion Diagnostic Devices. The FDA has approved the VENTANA MMR RxDx panel as a companion diagnostic assay to determine eligibility for dostarlimab-gxly Jemperli as.
47 Zeilen In addition the use of an IVD companion diagnostic device is stipulated in. FDA approved companion diagnostics In 2014 the FDA issued a regulatory guidance document on CDx which defines this type of assay as an in vitro diagnostic device IVD that provides information that is essential for the safe and effective use of a corresponding therapeutic product. Companion diagnostics are medical devices that help doctors decide which treatments to offer patients and which dosage to give tailored specifically to the patient says Elizabeth A.
FDA expects that most therapeutic product and IVD companion diagnostic device pairs will not meet the definition of combination product under 21 CFR 32e. The regulatory agency also allowed for a label expansion for the test to report additional select copy. Backed by our team of medical and scientific subject matter experts across all therapeutic areas youll.
As a Contract Diagnostics Organization CDO ResearchDx is a unique integrated business entity that solves logistical complexities associated with multi-partner outsourcing for regulated companion diagnostic development. Navigating the development path for FDA-approved companion diagnostics doesnt have to be complex it just requires a partner with expertise experience and resources that knows the way. The FDAs regulatory framework for companion diagnostics was finalized in 2014 Lee notes.
We supported the development of the first two FDA-approved complementary diagnostics and can help you weigh your strategic options. Specifically the guidance intends to accomplish the following. This guidance describes considerations for the development and labeling of in vitro companion diagnostic devices referred to as companion diagnostics herein to support the indicated uses of.
Approval policies stipulate that the therapeutic product and diagnostic test should cross-reference each other in labeling and that the two products be approved simultaneously although there are exceptions for drugs that treat a life-threatening condition. In October 2020 the FDA approved the FoundationOne Liquid CDx assay for use as a companion diagnostic for 3 targeted therapies in several tumor types. Guardant360 CDx Receives FDA Approval as Companion Diagnostic for Janssens RYBREVANT amivantamab-vmjw for Use in Patients with Advanced Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion.
Companion Diagnostics Definition auf Basis der FDA USA Companion Diagnostics soll dienen zur Identifikation solcher Patienten die erfolgreich und sicher mit einer bestimmten Behandlung therapiert werden können Identifikation solcher Patienten bei denen ein hohes Risiko ernster.
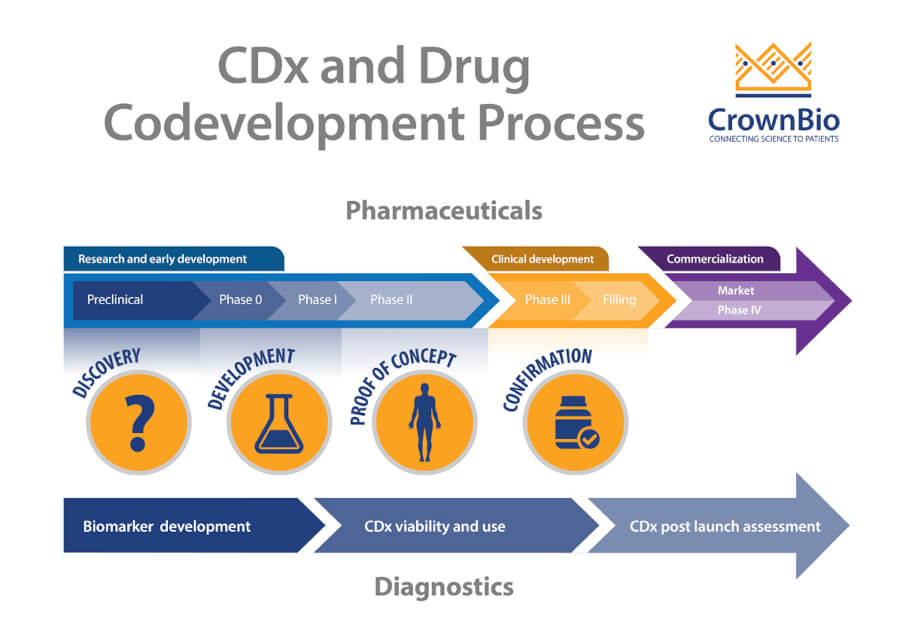
 Challenges In The Codevelopment Of Companion Diagnostics Personalized Medicine
Challenges In The Codevelopment Of Companion Diagnostics Personalized Medicine
 Overview Of Companion Diagnostics In The Pharmaceutical Industry Part 1 Drug Discovery World Ddw
Overview Of Companion Diagnostics In The Pharmaceutical Industry Part 1 Drug Discovery World Ddw
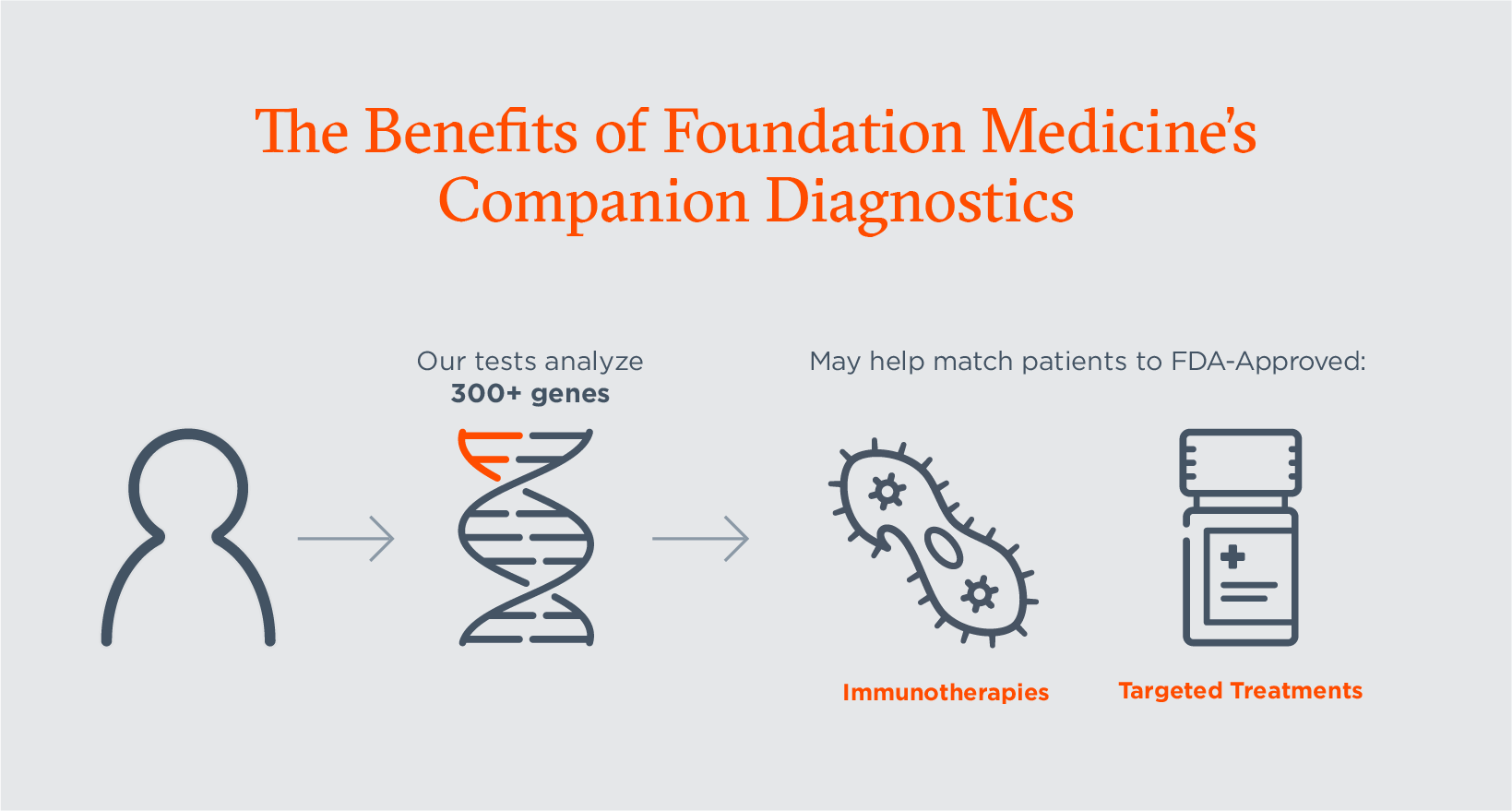 Companion Diagnostics Explained Their Critical Role In Cancer Care And Our Latest Approvals Foundation Medicine
Companion Diagnostics Explained Their Critical Role In Cancer Care And Our Latest Approvals Foundation Medicine
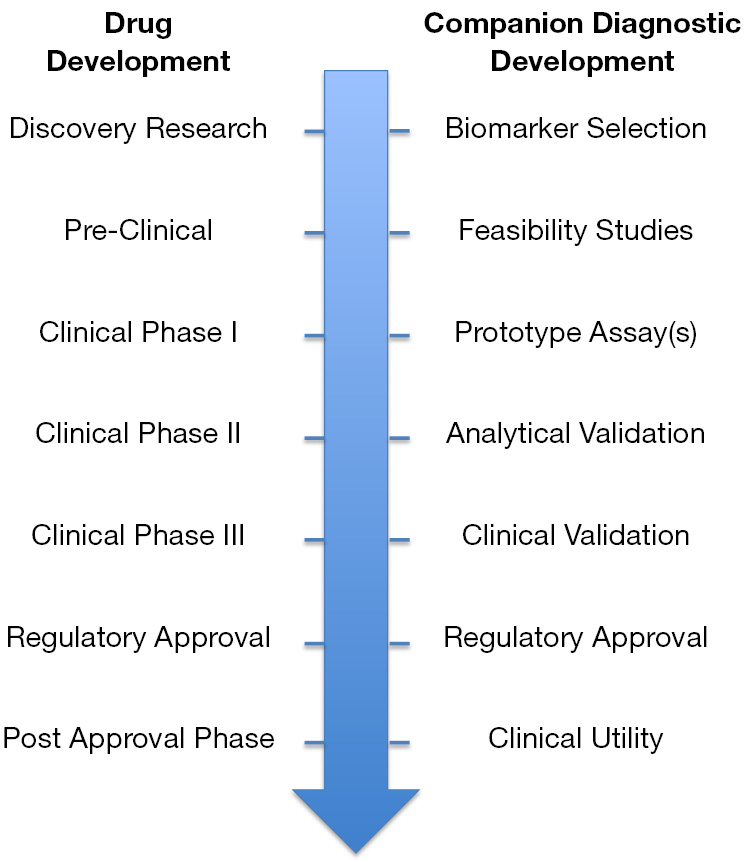 Companion Diagnostics A Tool To Improve Pharmacotherapy Jorgensen Annals Of Translational Medicine
Companion Diagnostics A Tool To Improve Pharmacotherapy Jorgensen Annals Of Translational Medicine
 Clinical And Regulatory Aspects Of Companion Diagnostic Development In Oncology Jorgensen 2018 Clinical Pharmacology Amp Therapeutics Wiley Online Library
Clinical And Regulatory Aspects Of Companion Diagnostic Development In Oncology Jorgensen 2018 Clinical Pharmacology Amp Therapeutics Wiley Online Library
 Companion Diagnostics From A Business Perspective Mddionline Com
Companion Diagnostics From A Business Perspective Mddionline Com
 Fda Approved Companion Diagnostics Cdx Download Table
Fda Approved Companion Diagnostics Cdx Download Table
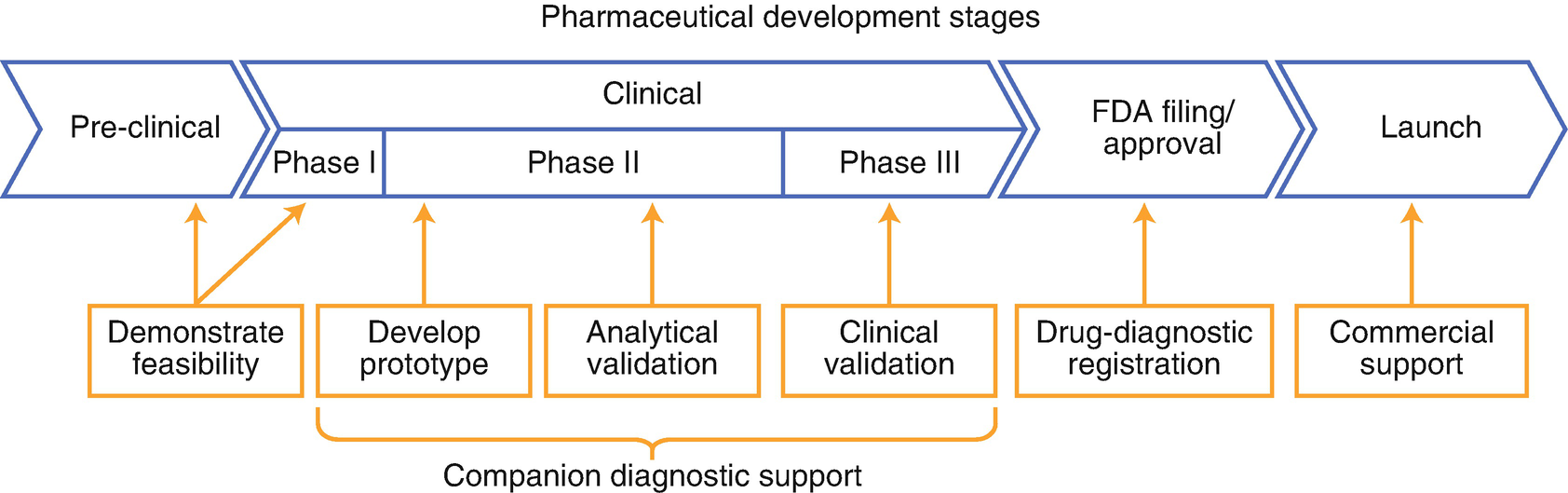 Use Of Companion Diagnostics Cdx And Predictive Biomarkers For Cancer Targeted Therapy Clinical Applications In Precision Medicine Springerlink
Use Of Companion Diagnostics Cdx And Predictive Biomarkers For Cancer Targeted Therapy Clinical Applications In Precision Medicine Springerlink
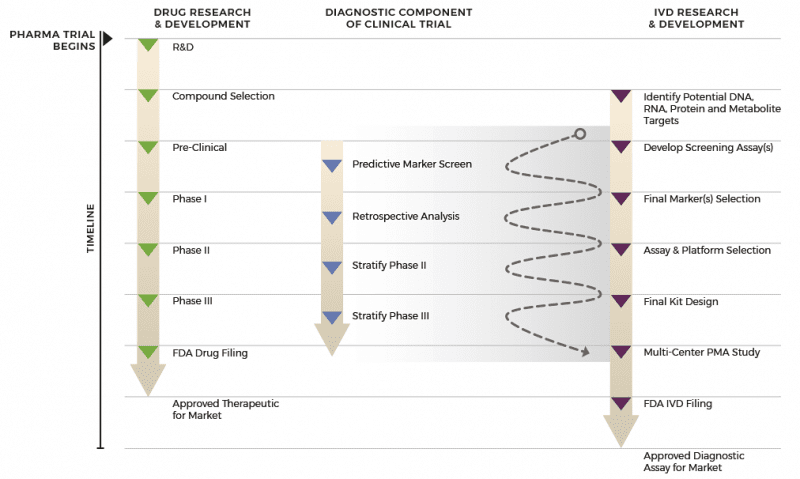 Fda Approved Companion Diagnostics Researchdx
Fda Approved Companion Diagnostics Researchdx
 Companion And Complementary Diagnostics Clinical And Regulatory Perspectives Trends In Cancer
Companion And Complementary Diagnostics Clinical And Regulatory Perspectives Trends In Cancer
 Companion Diagnostic Development Precision For Medicine
Companion Diagnostic Development Precision For Medicine
 Fda Approved Companion Diagnostics Cdx Download Table
Fda Approved Companion Diagnostics Cdx Download Table


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.